The Need for a Better Model of Multiple Myeloma
Multiple Myeloma (MM) is the second most prevalent hematological malignancy and causes approximately 20% of all deaths due to hematological malignancies (1). Newly diagnosed MM patients with standard-risk disease are associated with a median overall survival of about 4 years, whereas high-risk disease MM patients are associated with a median overall survival of about 2 years. Despite the significant efficacy of novel therapies to eradicate MM in vitro and in animal models, many MM patients (especially in relapsed setting) fail to respond to therapy (2).
Problems with Traditional Drug Discovery Models
The discrepancy between high success rates with laboratory models and a lack of clinical efficacy may be due to: (a) use of cultures of cancer cell line mono-cultures, excluding the essential bone marrow microenvironment; (b) failure to recreate the 3D tissue-like structure of the bone marrow niche, with corresponding oxygen and drug gradients; and (c) dependence upon a small number of uniform cell lines which cannot capture patient heterogeneity (1).
Our Novel 3D Tissue Engineered Bone Marrow (3DTEBM) Model
We developed a novel patient-derived 3D tissue engineered bone marrow (3DTEBM) model solely using patient material, with no additional exogenous material. It is created through the crosslinking of Fibrinogen, which is naturally found in the BM supernatant. This includes all of the accessory cells in the bone marrow microenvironment, as well as the MM cells (3). The 3DTEBM can demonstrate the interactions of cancer cells with the malignant microenvironment and recreate the 3D aspects of the BM niche. As a result, the 3DTEBM can model individual patient heterogeneity in progression and response to therapy (1).
Engineering the Bone Marrow Microenvironment

3DTEBM cultures promote MM cell proliferation better than 2D and commercially available 3D systems: Confocal microscopy images of a 3DTEBM seeded with MM cells (GFP – green), stromal cells (DiD – red), and endothelial cells (DiI – blue). At first, the cells form a homogeneous culture. However by day 7, the endothelial cells have migrated to the top, the stroma to the bottom, and the MM cells remain spread throughout the 3D (3).
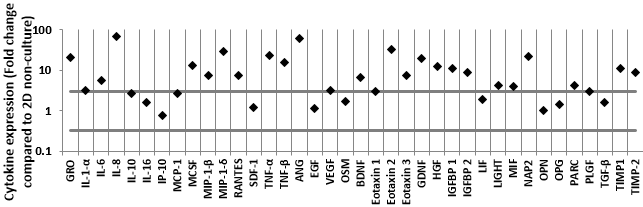
The 3DTEBM cultures induce changes in cytokine secretion in the MM environment: The baseline profile of cytokines was tested in the different cultures without the presence of cells. The cytokine profile of 3DTEBM non-cultured (BM supernatant) compared to 2D non-cultured (cell culture media) revealed that 23 of 40 detectable cytokines were enriched in the 3DTEBM with at least 3-fold increase compared to 2D tissue cultures (3).
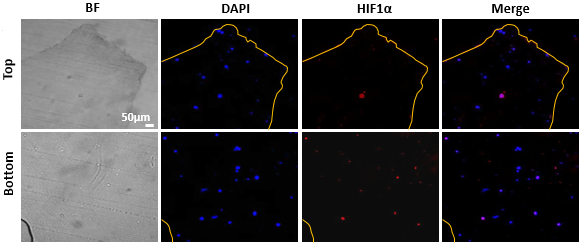
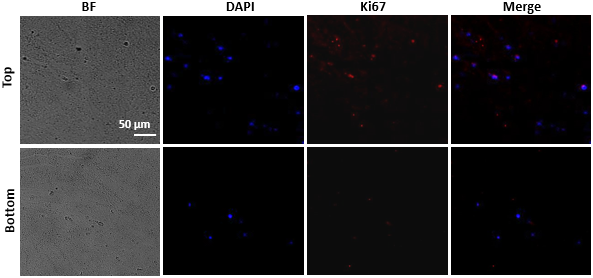
The oxygen gradients in the 3DTEBM affect cell proliferation: Fluorescent imaging at day 5 of the top and bottom areas of 3DTEBM cut longitudinally. Quantification of the Ki67 signal, a cellular proliferation marker, revealed a significantly higher number of proliferating MM cells within the top layers of the 3DTEBM compared to the bottom layers. Quiescent MM cells were found predominantly in the bottom layers, while more proliferative cells (higher Ki67 expression) were found near the top layers of the 3DTEBM scaffold (3).
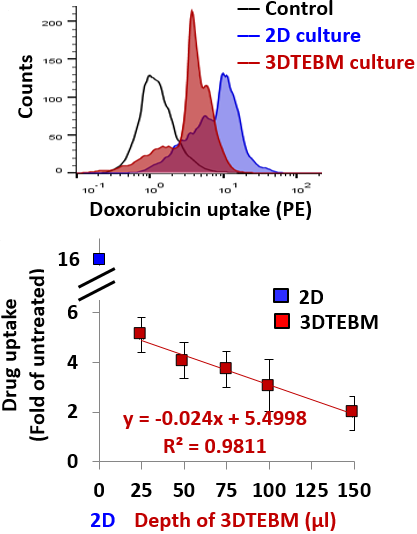
The 3DTEBM cultures recapitulate drug gradients: Flow cytometry histogram representing doxorubicin uptake (PE signal) in MM cells grown in 3DTEBM and 2D cultures. This shows the reduced doxorubicin uptake in 3DTEBM compared to 2D mono-cultures (3).
The 3DTEBM cultures recapitulate drug gradients: Correlation between various 3DTEBM and doxorubicin uptake of MM cells grown within. The blue square indicates doxorubicin uptake in 2D cultures (minimal depth) and the red squares show uptake in various 3DTEBM scaffold depths. The drug uptake of MM cells grown in 3DTEBM was inversely correlated with the depth of the scaffold (3).
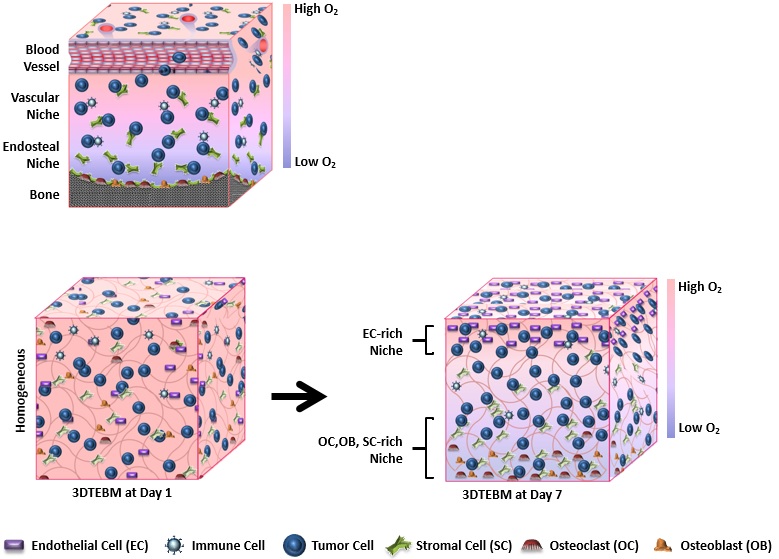
Structural resemblance of the 3DTEBM culture to the bone marrow niche in multiple myeloma: Some cells, like endothelial cells, prefer an oxygen-rich environment while others, like osteoclasts, prefer a hypoxic environment. Different cell types in the 3DTEBM redistribute themselves according to their affinity for oxygen and, after one week, the model evolves from a homogenous mixture to heterogenous bone marrow niche (1).
Unlike any other available 3D-cell culture system, the 3DTEBM allows the proliferation of primary MM cells ex vivo.
Applications of the 3DTEBM Model
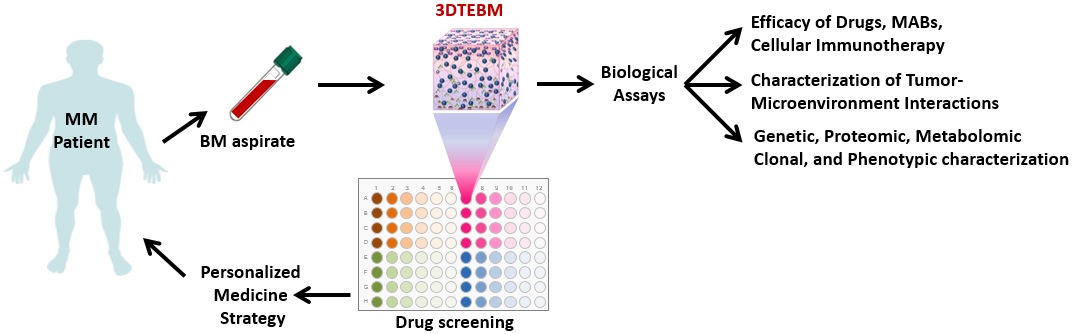
The 3DTEBM technology provides an improved model for studying cancer biology and drug resistance within the malignant BM microenvironment (1). Our model can be used as an ex vivo comparison to MM patient outcomes, particularly in clinical trials. The 3DTEBM is also an excellent model for use in drug development. Future applications would be to provide personalized prediction of therapeutic efficacy in individual MM patients. Current studies are being conducted to expand the use of the 3DTEBM to other hematologic malignancies and solid tumors (2, 4).
References
-
Azab, A. K. “Perspective of 3-D tissue-engineered bone marrow.” Medicographia 38.4 (2016): 387-93.
-
de la Puente, Pilar, and Abdel Kareem Azab. “3D tissue-engineered bone marrow: what does this mean for the treatment of multiple myeloma?.” (2016): 1545-1547.
-
de la Puente, Pilar, et al. “3D tissue-engineered bone marrow as a novel model to study pathophysiology and drug resistance in multiple myeloma.” Biomaterials 73 (2015): 70-84.
-
Azab, Abdel Kareem. “3-D Technology.” Genome (2016): 57.
